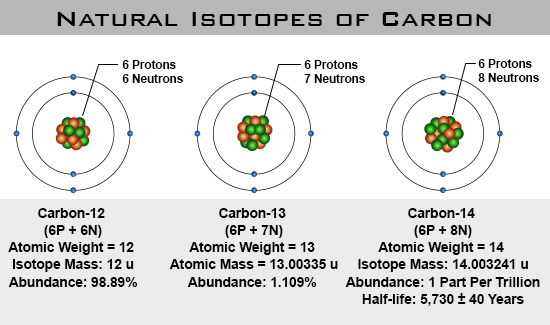
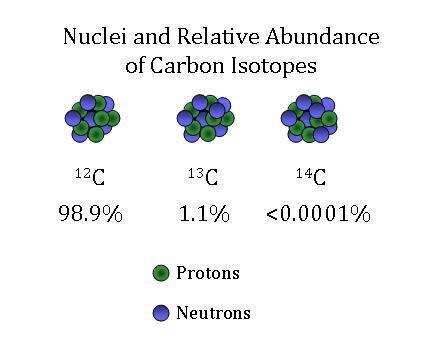
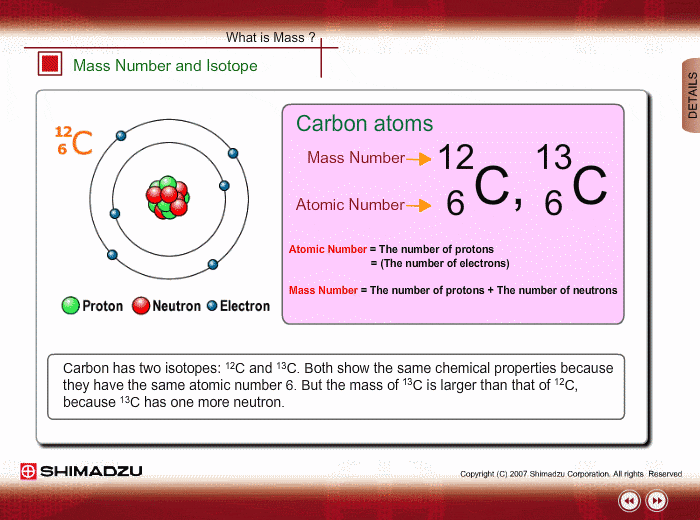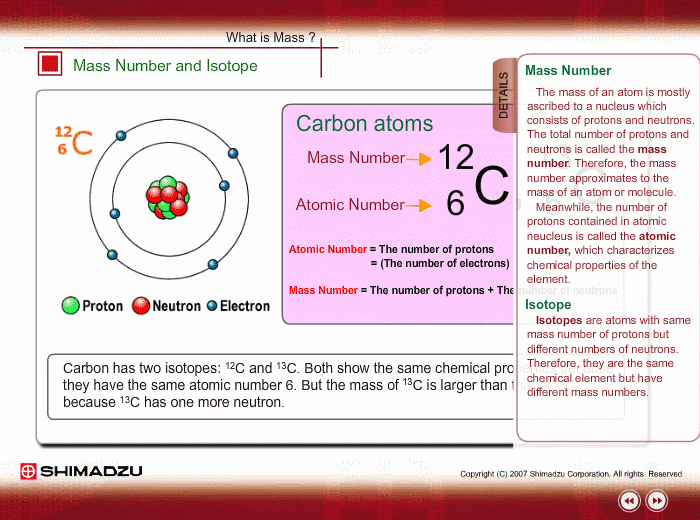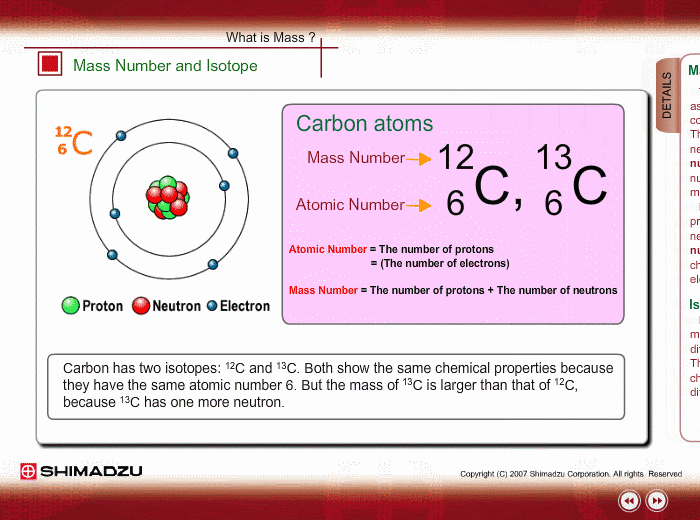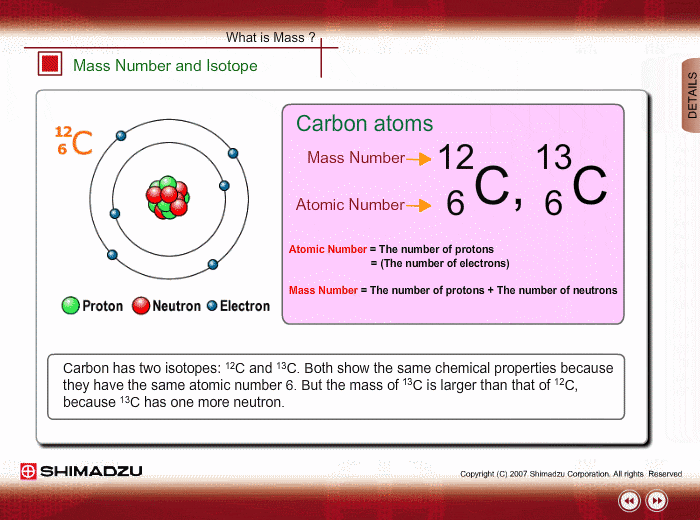
One Isotope of Carbon Has Exactly the Same Mass Number
Here Nᵒ represents the number of atoms of the isotope in the sample at t0 or when the organism a part of whom now forms the sample died while N represents the number of atoms left after time t has passed. For 12 C the atomic mass is exactly 12u since the atomic mass unit is defined from it.
5 Carbon Introduction To Climate Science
For some isotope mass difference is 1.
. So there will be some fraction of molecules that will be heavier than expected parent mass. The equation dictates the decay of a radioactive isotope. These differences are exhibited as multiple peaks in mass spec.
For example 63 Cu 29 protons and 34 neutrons has a mass number of 63 and an isotopic mass in its nuclear ground state is 6291367 u. Remember that the ratio of C-14 to C-12 atoms in the organism and the environment is the same when it is. For other isotopes the isotopic mass usually differs and is usually within 01 u of the mass number.
For others mass difference is 1. Every atom in a molecule has a chance of being one of these isotopes.

Atomic Mass Standard Mass Unit Is Derived From Carbon 12 Atomic Mass Unit The Mass Equal To 1 12 The Mass Of One Carbon 12 Atom Ppt Download

Chapter 4 The Atom Part 2 Subatomic Particles Particlesymbolchargemass Amu Location Electrone E 0 0 Orbit Nucleus Protonp P 1 1 1 Inside Nucleus Ppt Download
No comments for "One Isotope of Carbon Has Exactly the Same Mass Number"
Post a Comment